Crystallization is the separation process by which heat and mass transfer remove solids from a solution in the form of high purity crystalline structures. The two-part process of associated nucleation and crystal growth depends upon the relative rate of each step to control crystal size and population.
Found in a variety of process and waste management operations, crystallization also reduces liquid waste run-off, as the remaining liquid — also purified — can then be cycled back through the system, released into the environment, or passed on to another operation.Optimizing Crystallization Systems
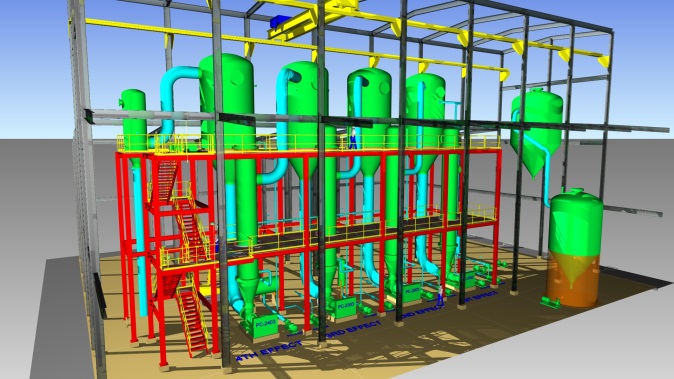
Thermal Kinetics Crystallizers
There are two main types of crystallizers: evaporative and cooling.Evaporative Crystallizers
Evaporative crystallization increases the concentration of the solution by evaporating the solvent. As the concentration increases, the solution becomes supersaturated and nucleation begins. Upon further concentration, the nuclei begin to grow and distinct crystals appear. Thermal Kinetics offers two main styles of evaporative crystallizers, each available with customization options:- Draft Tube Crystallizers — A continuous vacuum evaporating crystallizer, this model utilizes a low-speed propeller to direct the mixture upward through a draft tube toward the boiling surface to be evaporated. A small degree of supercooling minimizes the buildup of crystals on the walls, while the remaining liquid flows back downward through the draft tube. This crystallizer is useful for small capacity applications with moderate control of crystal size. Crystal size and uniformity of size depend on external separation, fines destruction, and crystal population control.
- Submerge Circulating Crystallizers — A circulating batch crystallizer in which, if using external circulation, the solution is pushed through heat exchanger tubes at a high velocity, this model allows minimal crystal formation on the tubes. If using internal circulation, the solution is pushed by a propeller through a draft tube to circulate internally while a hot feed supplies evaporation energy. Both versions require agitation or circulation to ensure small, pure crystal formation. This type of crystallizer is useful for larger capacities with the same external control of size and size uniformity.
Cooling Crystallizers
Cooling crystallization, the process of crystallizing solutions based on solubility temperature dependence, is conducted through indirect heat transfer or directly under vacuum. These methods cause some of the solution to evaporate and then cool to a temperature in equilibrium with the reduced pressure. In essence, a small amount of the solvent flashes off the latent heat, subsequently lowering the temperature of the solution. While this process does cause a reduction in solvent, the crystallization is mainly due to the change in temperature of the solution. Decreasing the temperature supersaturates the solution, developing the nuclei and encouraging crystal growth to force the salt out of solution and crystallize. The cooling crystallization process is performed using one of three systems: vacuum cooling crystallizers, continuous cooling crystallizers, or scraped surface crystallizers.- Vacuum Cooling Crystallizers — Useful for maintaining tighter control of crystal size, this vessel utilizes either a batch or a continuous crystallization process. The batch operation is optimal for controlled crystal sizing, as each crystal endures the process for the same amount of time, leading to consistent dimensions.
- Continuous Cooling Crystallizers — This model removes a slurry to a centrifuge, returns centrifuged dilute solution to an evaporator for concentration, and then supplies this concentrated warm solution to the cooling crystallizer.
- Scraped Surface Crystallizers — Once its chilled vessel walls cause the solution to crystallize on the wall surface, this model uses blades in rotation against the walls to dislodge the formed crystals. Once collected, the crystals are returned back to the bulk of the solution for growth and ultimate extraction as a slurry for further separation in a centrifuge.
Full-Service Crystallizer Support
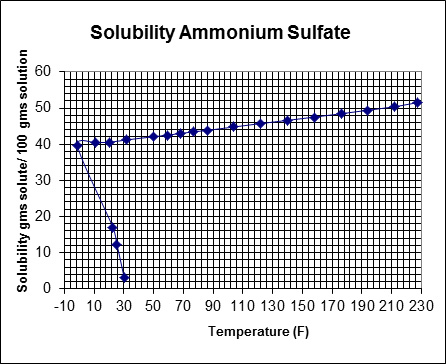
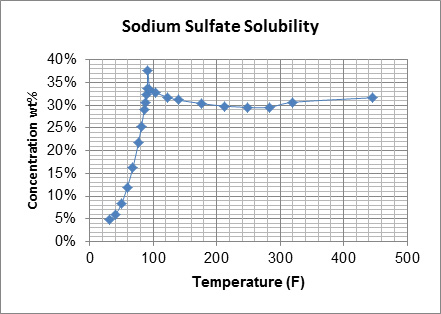
Thermal Kinetics also supplies the supporting equipment that goes with each crystallizer option. This includes centrifuges to separate the crystals from the saturated solution, splitter boxes/vessels for fines control and destruction, and control valves for the process, pumps, tanks, and instrumentation. Hydroclones are often employed to aid in the control of crystal size, return of fines to the crystallizer, and thicken the slurry prior to centrifuging. We also offer modular system design and support, allowing for the pre-assembly of all system components on a modular skid package for off-site manufacture and seamless on-site installation, depending on equipment size and location. Equipped with a database of prior crystallization projects to inspire design approaches and applications, along with testing facilities to gather physical data on your crystallization, the Thermal Kinetics team is well positioned to support your plant’s system needs. This is especially useful for proprietary and specialty chemicals for which solubility data is not readily available, or for a solution of various chemicals which affects the solubility of involved salts.Partner with Thermal Kinetics
By using a single supplier to engineer, procure, and assemble the entire new system, clients lower and control project costs, improve job coordination and oversight, and ensure peace of mind. To learn more about how our crystallization equipment and design capabilities can assist in your next project, contact a member of the Thermal Kinetics team today.All Products
-
Fuel Ethanol PlantsFuel Ethanol Plants
-
Adsorption EquipmentAdsorption Equipment
-
Evaporation EquipmentEvaporation Equipment
-
Industrial Distillation EquipmentIndustrial Distillation Equipment
-
Crystallizer EquipmentCrystallizer Equipment
-
Specialty Process SystemsSpecialty Process Systems
-
Modular SystemsModular Systems